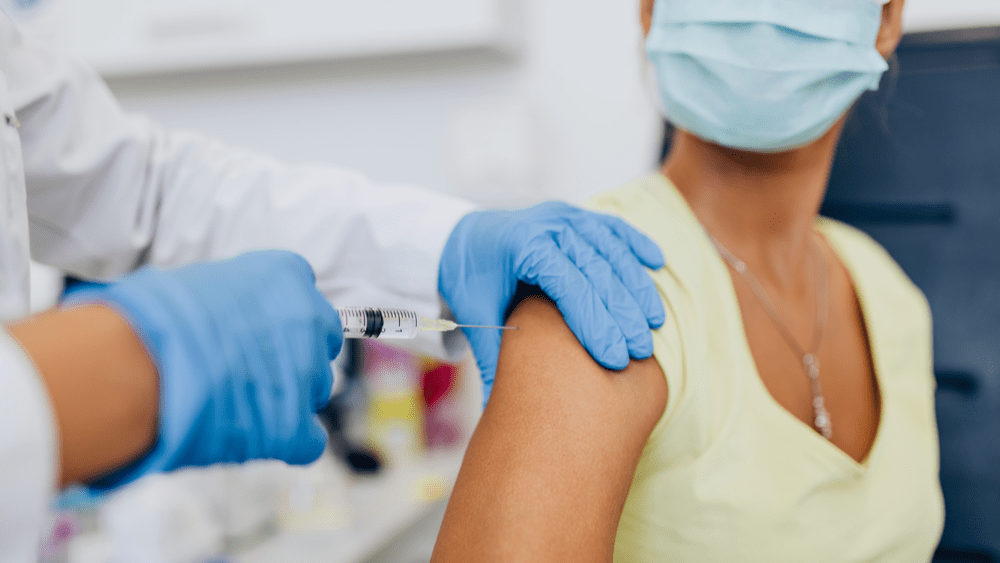
Johnson & Johnson submitted an application on Thursday to the Food and Drug Administration for an emergency use authorization of its experimental Covid-19 vaccine. The FDA could grant that authorization within weeks; if authorized, it would give the U.S. a third Covid-19 vaccine, along with those from Pfizer-BioNTech and Moderna.
Dr. Paul Stoffels, chief scientific officer at Johnson & Johnson, said in a press release, “Today’s submission for emergency use authorization of our investigational single-shot Covid-19 vaccine is a pivotal step toward reducing the burden of disease for people globally and putting an end to the pandemic.” Stoffels said the company would be ready to begin shipping vaccines if the FDA grants the company emergency use authorization. J&J previously said it expects to supply 100 million doses to the U.S. by June. The Johnson & Johnson vaccine, made in partnership with Janssen Pharmaceuticals, only requires basic refrigeration and is given as a single dose. Other vaccines, including Pfizer and Moderna’s shots, require two doses.